Fourier Transform InfraRed Spectroscopy
The goal of any absorption spectroscopy (FTIR, ultraviolet-visible ("UV-Vis") spectroscopy, etc.) is to measure how well a sample absorbs light at each wavelength. The most straightforward way to do this, the "dispersive spectroscopy" technique, is to shine a monochromatic light beam at a sample, measure how much of the light is absorbed, and repeat for each different wavelength. (This is how UV-Vis spectrometers work, for example.)
Fourier transform spectroscopy is a less intuitive way to obtain the same information. Rather than shining a monochromatic beam of light at the sample, this technique shines a beam containing many different frequencies of light at once, and measures how much of that beam is absorbed by the sample. Next, the beam is modified to contain a different combination of frequencies, giving a second data point. This process is repeated many times. Afterwards, a computer takes all this data and works backwards to infer what the absorption is at each wavelength.
The beam described above is generated by starting with a broadband light source—one containing the full spectrum of wavelengths to be measured. The light shines into a certain configuration of mirrors, called a Michelson interferometer, that allows some wavelengths to pass through but blocks others (due to wave interference). The beam is modified for each new data point by moving one of the mirrors; this changes the set of wavelengths that pass through.
As mentioned, computer processing is required to turn the raw data (light absorption for each mirror position) into the desired result (light absorption for each wavelength). The processing required turns out to be a common algorithm called the Fourier transform (hence the name, "Fourier transform spectroscopy"). The raw data is sometimes called an "interferogram".
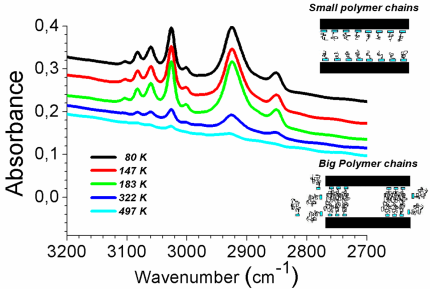 Traditionally, FTIR spectra of
PS-PEO block copolymers end-adsorbed onto porous alumina of 200 nm mean pore
size from an incubating solution at a concentration of 0.5 mg/mL in toluene.
Adsorption time: 24 h. The adsorbed amount drops sharply for the high molecular
weight polymers. The spectra have been offset for reasons of clarity.
Traditionally, FTIR spectra of
PS-PEO block copolymers end-adsorbed onto porous alumina of 200 nm mean pore
size from an incubating solution at a concentration of 0.5 mg/mL in toluene.
Adsorption time: 24 h. The adsorbed amount drops sharply for the high molecular
weight polymers. The spectra have been offset for reasons of clarity.